titanium electron configuration|Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and : Baguio Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high .
7-axis CNC machining is a sophisticated manufacturing process that utilizes seven axes of movement. This allows for the creation of complex geometries and parts with tight .
titanium electron configuration,Learn the electron configuration of titanium, a transition metal with a gray tint and high corrosion resistance. Find out its physical and chemical properties, common compounds and extraction methods. Mar 23, 2023 Learn how to write the electron configuration of titanium (Ti) through orbitals or orbitals, using Bohr's principle or Aufbau principle. See the video, diagrams, .titanium electron configuration Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number of electrons for the Ti atom.

Titanium electron configuration. ← Electronic configurations of elements. Ti (Titanium) is an element with position number 22 in the periodic table. Located in the IV period. .
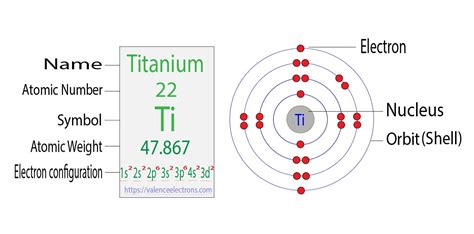
Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high .Titanium is a hard, shiny and strong metal with the electron configuration [Ar] 3d 2 4s 2. It is used in many alloys, pigments, coatings and surgical implants. This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble.Titanium is a transition metal with the electron configuration [Ar] 3d 2 4s 2. Learn about its history, crystal structure, physical and chemical properties, applications, isotopes and more on this web page.Titanium is a transition metal with symbol Ti and atomic number 22. Its electron configuration is [Ar] 3d 2 4s 2, with valence electrons 2, 3, 4.
The excited state of titanium electronic configuration is 1s2 2s2 2p2 3s2 3p6 3d2 4s1. . Ground state Titanium orbital diagram. The ground state titanium orbital configuration is 1s 2 2s 2 2p 2 3s 2 3p 6 3d 2 4s 2 .The s can hold two electrons. The p subshell has three orbitals. Each can carry two electrons. If the electron configuration of titanium was 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4, four of the five 3d orbitals would contain one electron each. And the 4s orbital would be unoccupied. In Figure 6b, the 3d and 4s .Element Titanium (Ti), Group 4, Atomic Number 22, d-block, Mass 47.867. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and . To write the orbital diagram for the Titanium (Ti) first we need to write the electron configuration for just Ti. To do that we need to find the number of e. To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell.The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule.
Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble. Therefore, the complete electron configuration of titanium (Ti 2+) ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 and the unabbreviated electron configuration of titanium (Ti 2+) ion is[Ar] 3d 2. This electron configuration shows that the titanium (Ti 2+) ion has three shells and the last shell has eight electrons.Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 2 4s 2; Electrons per Energy Level: 2,8,10,2 . Titanium - Ti (EnvironmentalChemistry.com)- Comprehensive information for the element Titanium - Ti is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked .

Titanium. Full electron configuration of titanium: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2 scandium ← titanium → vanadium. © 2009-2016 | www.prvky.com | kontaktkontakttitanium electron configurationTitanium. Full electron configuration of titanium: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2 scandium ← titanium → vanadium. © 2009-2016 | www.prvky.com | kontaktkontaktMit der Elektronenkonfiguration jedes Elements lässt sich angeben, wie die Elektronen in den Atomen dieses Elements aufgebaut sind. Im Fall von Titan beträgt sein mittlerer Radius 140 pm, sein kovalenter Radius 136 pm, sein Bohr-Radius oder Atomradius 176 pm. Insgesamt hat Titan 22 Elektronen, seine Verteilung ist wie folgt: Die erste Schicht hat 2 .La configuration électronique du titane est 1s2 2s2 2p6 3s2 3p6 4s2 3d2. Le titane est appelé l'élément chimique dont le numéro atomique est 22, son symbole est Ti, il fait partie du tableau périodique des éléments et fait partie du groupe 4.Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation .
titanium electron configuration|Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and
PH0 · Titanium Electron Configuration (Ti) with Orbital Diagram
PH1 · Titanium (Ti)
PH2 · Titanium
PH3 · Orbital Diagram of Titanium (Ti), electron configuration, and
PH4 · Electron configuration of titanium
PH5 · Electron configuration for Titanium (element 22). Orbital diagram
PH6 · Electron Configuration for Titanium (Ti2+,Ti3+,Ti4+ ions)
PH7 · Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and
PH8 · Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and
PH9 · Electron Configuration Chart of All Elements (Full Chart)